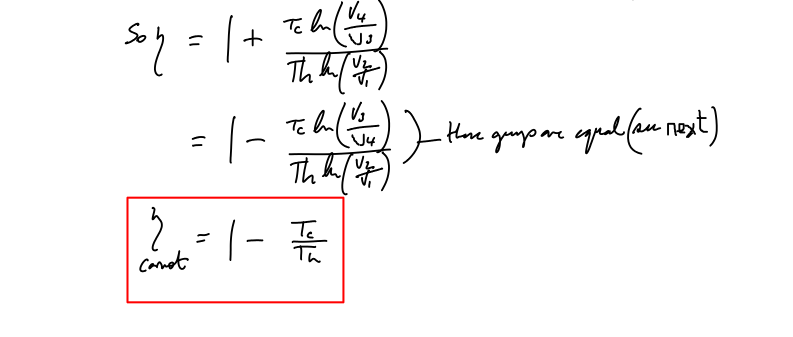Here I show how the Clausius inequality points towards a new property of state: S
Category: Thermo
Thermodynamics notes
The Clausius inequality
Before talking about entropy, easily the most confusing aspect of engineering thermodynamics, it’s important to get clear what the Clausius inequality is. Here, again, I insist that heat transfers into a system will be taken as positive and those in which heat leaves a system are negative in value (these I label eg dQ(-) ).
Thermodynamic Kelvin temperature = Ideal gas Kelvin temperature
A temperature scale based on heat engine behaviour only
A thermodynamic definition for when T1>T2
When an engine runs between T1 and T2, ht from T2 to T1 is impossible
Why can’t we have a 100%-efficient engine?
Proof that V2/V1=V3/V4 for the Carnot cycle
As stated in an earlier section, here is the required proof. I’ve often seen this ‘left as an exercise for the reader’. Such exercises are fine -once you understand the material but not as part of the explanation. One danger is that those who are less fluent with mathematical techniques are left wondering where all this stuff comes from…