Note here the reference to ‘one-way work transfer’. Work is not a process in itself, but an exchange of energy (which may form part in a process). It therefore makes little sense to talk about reversible or irreversible work. Nonetheless there are recognisable differences between, say, the work that is done by a stirrer and the work that is done by a piston in quasi-equilibrium.
Note here the reference to ‘one-way work transfer’. Work is not a process in itself, but an exchange of energy (which may form part in a process). It therefore makes little sense to talk about reversible or irreversible work. Nonetheless there are recognisable differences between, say, the work that is done by a stirrer and the work that is done by a piston in quasi-equilibrium.
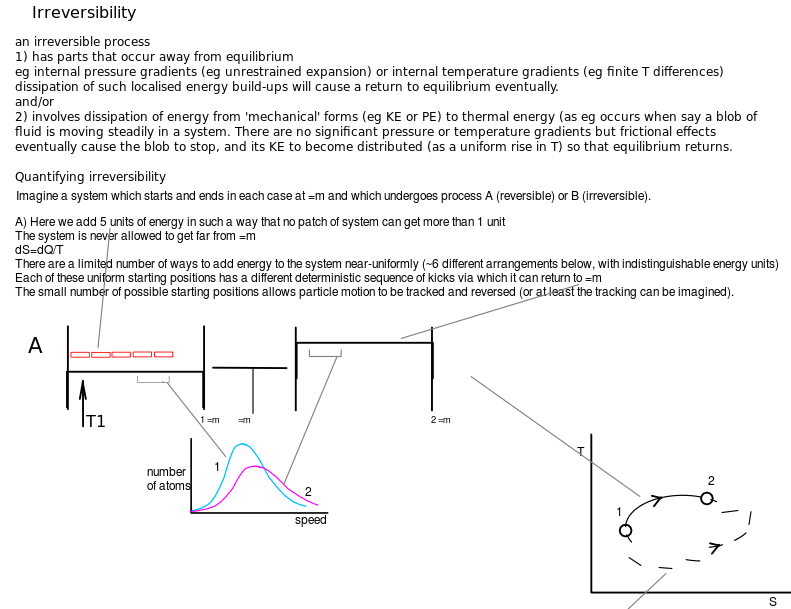
Stirrer work is a transfer of energy which results in dissipation (change of energy from mechanical, bulk or coordinated motion to the thermal (pseudorandom) motion of particles).
Allowing the stirrer freedom to rotate in the opposite direction does not cause energy extraction from the stirred system, however. The stirrer shaft does not spontaneously rotate in the opposite direction and unstir the system contents (hence the term one-way).
Quasi-equilibrium piston work causes zero dissipation. Allowing the piston motion to reverse direction (by reducing the force on the piston to a level infinitesimally less than the force due to internal pressure) does cause the extraction of energy from the compressed system, hence the term two-way work.
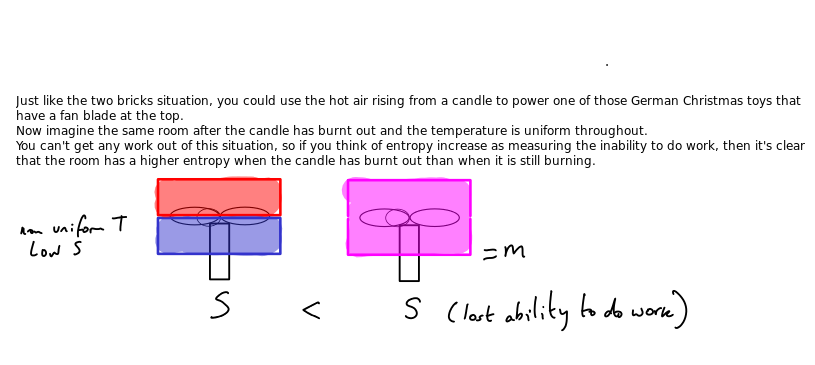
I have already talked about the heat transfer aspect of reversibility. The diagram above is correct but does not explain the situation clearly enough.